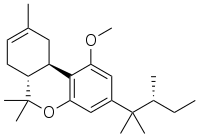
JWH-359
In this article, we will delve into the world of JWH-359, exploring its many facets and manifestations. From its origins to its relevance today, JWH-359 has captured the attention of people of all ages and backgrounds. Through this comprehensive analysis, we will seek to better understand what makes JWH-359 so fascinating and what impact it has on our society. Regardless of whether you are an expert on the subject or simply curious to learn more, this article will provide you with the information necessary to fully understand the phenomenon of JWH-359. Get ready to immerse yourself in a journey of discovery and knowledge!
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H36O2 |
| Molar mass | 356.550 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
JWH-359 is a dibenzopyran "classical" cannabinoid drug, which is a potent and selective CB2 receptor agonist, with a Ki of 13.0 nM and selectivity of around 220 times for CB2 over CB1 receptors. It is related to other dibenzopyran CB2 agonists such as JWH-133 and L-759,656 but with a chiral side chain which has made it useful for mapping the shape of the CB2 binding site. It was discovered by, and named after, John W. Huffman.
References
- ^ Huffman JW, Bushell SM, Joshi SN, Wiley JL, Martin BR (January 2006). "Enantioselective synthesis of 1-methoxy- and 1-deoxy-2'-methyl-delta8-tetrahydrocannabinols: new selective ligands for the CB2 receptor". Bioorganic & Medicinal Chemistry. 14 (1): 247–62. doi:10.1016/j.bmc.2005.08.013. PMID 16165365.
- ^ Marriott KS, Huffman JW (2008). "Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor". Current Topics in Medicinal Chemistry. 8 (3): 187–204. doi:10.2174/156802608783498014. PMID 18289088.